
In the dark, cGMP levels are high and keep cGMP-gated sodium channels open allowing a steady inward current, called the dark current. In photopic conditions (light), photoreceptors hyperpolarize to a potential of −60 mV. Photoreceptor cells are unusual cells in that they depolarize in response to absence of stimuli or scotopic conditions (darkness). Note that this is significantly more depolarized than most other neurons.Ī high density of Na +-K + pumps enables the photoreceptor to maintain a steady intracellular concentration of Na + and K +.
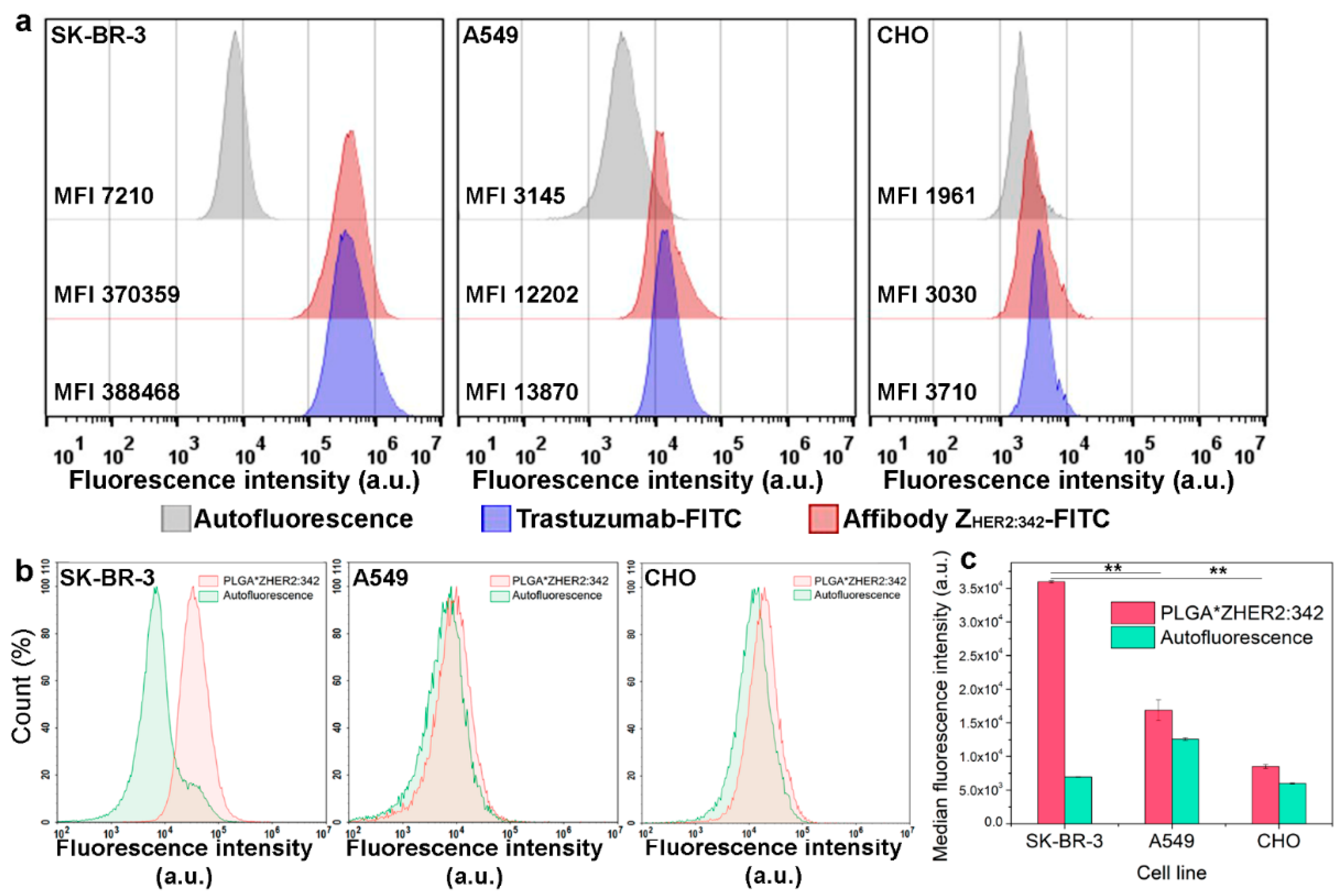
This " dark current" depolarizes the cell to around −40 mV. There is also an inward sodium current carried by cGMP-gated sodium channels. This outward current tends to hyperpolarize the photoreceptor at around −70 mV (the equilibrium potential for K +). There is an ongoing outward potassium current through nongated K +-selective channels. To understand the photoreceptor's behavior to light intensities, it is necessary to understand the roles of different currents. The absorption of light leads to an isomeric change in the retinal molecule. Finally, it is oxidized to 11- cis-retinal before traveling back to the photoreceptor cell outer segment where it is again conjugated to an opsin to form new, functional visual pigment ( retinylidene protein), namely photopsin or rhodopsin. The isomerase activity of RPE65 has been shown it is uncertain whether it also acts as the hydrolase. It is first esterified by lecithin retinol acyltransferase (LRAT) and then converted to 11- cis-retinol by the isomerohydrolase RPE65. When it absorbs a photon, 11- cis-retinal undergoes photoisomerization to all- trans-retinal, which changes the conformation of the opsin GPCR leading to signal transduction cascades which causes closure of cyclic GMP-gated cation channel, and hyperpolarization of the photoreceptor cell.įollowing photoisomerization, all- trans-retinal is released from the opsin protein and reduced to all- trans- retinol, which travels to the retinal pigment epithelium to be "recharged". The 11- cis-retinal is covalently linked to the opsin receptor via Schiff base. The visual cycle occurs via G-protein coupled receptors called retinylidene proteins which consists of a visual opsin and a chromophore 11- cis-retinal. Humans have trichromatic photopic vision consisting of three opponent process channels that enable color vision. The three types of cones are L-cones, M-cones and S-cones that respond optimally to long wavelengths (reddish color), medium wavelengths (greenish color), and short wavelengths (bluish color) respectively.
Each cone type responds best to certain wavelengths, or colors, of light because each type has a slightly different opsin.
#Photosensitive protein scaffold code
Cones, on the other hand, can code the color of an image through comparison of the outputs of the three different types of cones. Rods deal with low light level and do not mediate color vision. These cells contain a chromophore ( 11- cis-retinal, the aldehyde of vitamin A1 and light-absorbing portion) that is bound to a cell membrane protein, opsin. The photoreceptor cells involved in vertebrate vision are the rods, the cones, and the photosensitive ganglion cells (ipRGCs). Phototransduction in some invertebrates such as fruit flies relies on similar processes. It relies on the visual cycle, a sequence of biochemical reactions in which a molecule of retinal bound to opsin undergoes photoisomerization, initiates a cascade that signals detection of the photon, and is indirectly restored to its photosensitive isomer for reuse. Visual phototransduction is the sensory transduction process of the visual system by which light is detected to yield nerve impulses in the rod cells and cone cells in the retina of the eye in humans and other vertebrates.


 0 kommentar(er)
0 kommentar(er)
